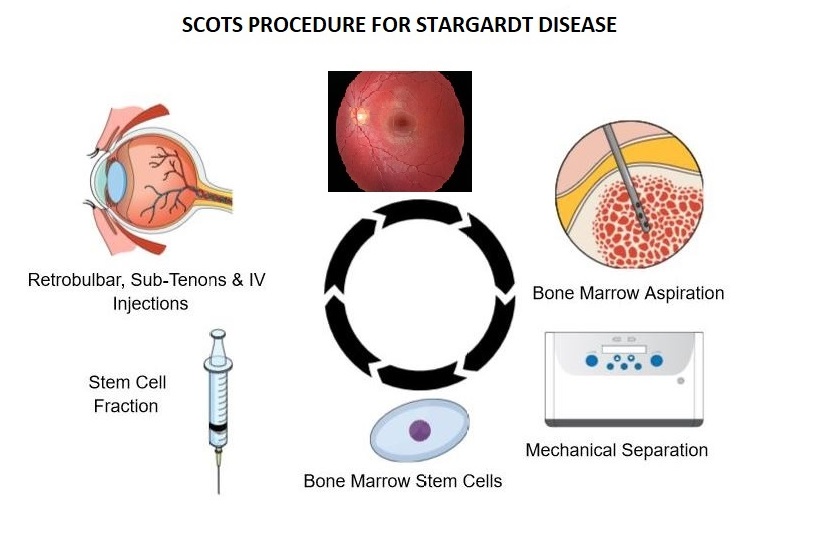
Stargardt Disease is the most common inherited macular degeneration, typically starting at a young age and progressively worsening to legal blindness. This can affect many activities in a person’s life, causing difficulty with reading, school, work, driving and seeing faces. Eye doctors have not been able to offer any potential treatment for improvement- until now. MD Stem Cells has just published results in the highly respected journal Medicines in a special issue: Stem Cells in Eye Research and Ophthalmology- Current Advances and Future Directions. The doctors conducting the Stem Cell Ophthalmology Treatment Study (SCOTS2) now have very encouraging data for patients with Stargardt Disease.
The Stem Cell Treatment Ophthalmology Study- both SCOTS and SCOTS2 is using the patient’s own bone marrow stem cells (BMSC) for treating optic nerve and retinal disease. The study is Institutional Board Approved and National Institutes of Health registered on www.clinicaltrials.gov NCT 03011541. A number of different optic nerve diseases including NAION, LHON, DOA, optic atrophy; as well as retinal diseases including Serpiginous, Cone-Rod, Retinitis Pigmentosa, Ushers, dry AMD and now Stargardt have shown benefit. MD Stem Cells has worked to ascertain the safest, most effective approach to Stargardt Disease using BMSC - with gratifying success.
In the 34 eyes treated, vision improved in 61.8% and remained stable in 23.5%- positive results in over 84% of eyes. As might be expected, some eyes continued to worsen as a result of the disease. The results were highly statistically significant- meaning that the treatment was overwhelmingly likely to be responsible for the vision benefit. The average improvement across all eyes treated was 17.96% (95% CI, 16.39 to 19.53%). Reviewing the overall results, 76.5% of patients showed improvement in one or both eyes, 17.6% showed no net loss and only 1 patient continued to worsen from their disease- so improvement or stability in over 94% of patients. This is similar to the results in dry Age Related Macular Degeneration.
“ This makes sense given that both Stargardt and dry AMD are primarily diseases of the macula or central part of the retina” says Dr. Levy, Chair and Study Director for SCOTS2. “Although dry AMD is does not typically start until late in life, and Stargardt is early, both affect the support layer of the retina called the Retinal Pigment Epithelium. Therefore, we can postulate that one way the Bone Marrow Derived Stem Cells (BMSC) that we are using can benefit the central vision is by helping this layer of the retina”.
SCOTS and now SCOTS2 have been evaluating BMSC in the treatment of optic nerve and retinal disease for over 7 years. The physicians involved with MD Stem Cells now have 15 world class medical and scientific publications primarily in ophthalmology, but also regarding their neurology study. This is vastly more than any other stem cell research group in this field- which is important to patients seeking experienced providers.
Dr. Levy concludes: “as we continue SCOTS 2, we can conclude that patients with Stargardt Disease choosing to participate in the study have a strong likelihood of either improving or stabilizing their vision. ”
About MD Stem Cells
Patients may receive information about SCOTS 2 by emailing This email address is being protected from spambots. You need JavaScript enabled to view it., using the contact us page on www.mdstemcellscom, or calling 203-423-9494. The Stem Cell Ophthalmology Study 2 is enrolling patients with different retina and optic nerve diseases. MD Stem Cells has no grant support and is not a pharmaceutical company; these are patient sponsored studies and the patients pay for both treatment and travel.